CAPA Example | Manufacturing examples | CAPA Meaning
Last updated on November 16th, 2024 at 08:42 am
CAPA Example | Manufacturing examples | CAPA Meaning
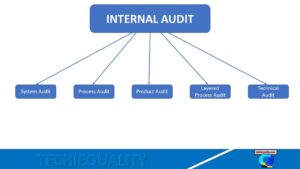
Hi Readers! Here, we are going to discuss on the most popular topic of the manufacturing industry is namely termed as CAPA Example. Firstly, we will be knowing details on CAPA meaning. The full form of CAPA is Corrective action and Preventive action. Where problems, issues, and any type of non-confirmative presence, there CAPA is required. In manufacturing units CAPA is most important and commonly used for problem solving and process improvement. Now we are going to discuss several manufacturing practical CAPA examples in different operations like quality, production, maintenance, purchasing, and HR functions.
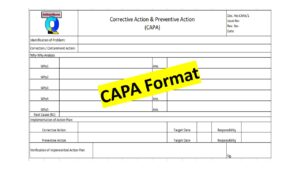
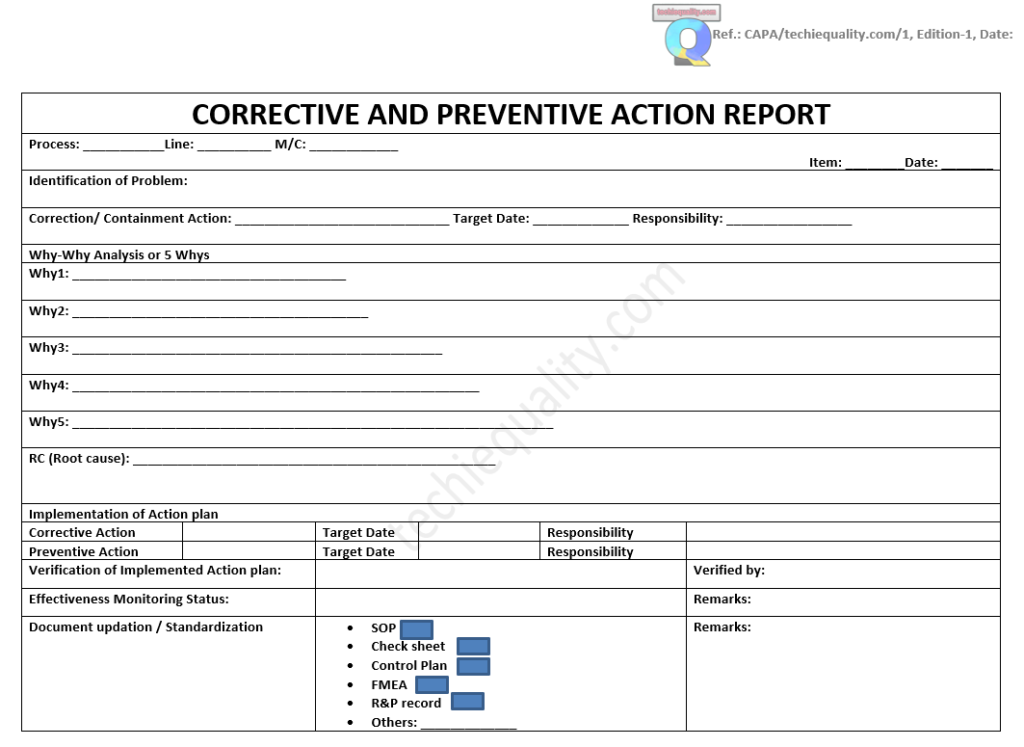
Download the CAPA Template or Format with an Example
CAPA Meaning:
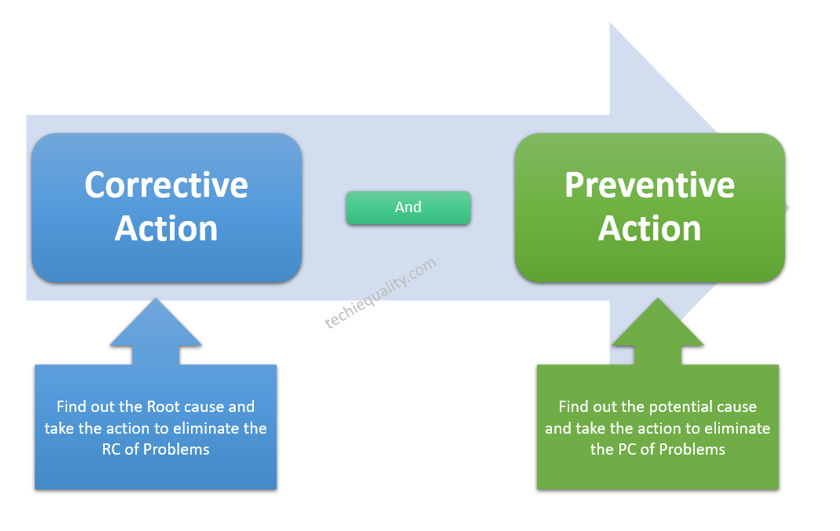
The CAPA means Corrective Action and Preventive Action. The action to eliminate the symptoms of the problem is called a correction. Action to eliminate the root cause of the problem is called corrective action. The action to eliminate the potential cause of the problem is called preventive action.
CAPA Example:
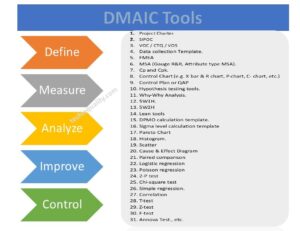
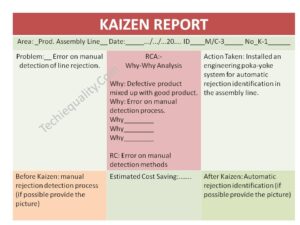
Practice makes you perfect, in line with this sentence, here we are going to practice some CAPA Example in Manufacturing Industry covering with different and different operations like quality activities, SRM, lab activities, purchase activities, etc. The main important part of the CAPA report is the root cause analysis, for doing the RCA the most commonly used tools are the why-why analysis or 5-whys analysis & the 2nd important part is systematic corrective and preventive action. CAPA report is commonly required where there are any issues, problems, risks, defects, warranty, customer complaints, & non-conformities.
Following are the steps that you can follow to write the CAPA report in simple ways.
- Identify the problem, issue or non-conformity.
- Define and describe the problem for a better understanding of the background of the problem.
- RCA -root cause analysis (common tools -why -why analysis or 5 whys)
- Action plan with a target date and responsibility (Correction, corrective action,, and preventive action, a common format is CAPA format or template, 8D report, etc.)
- Implement an action plan.
- Effectiveness monitoring.
- Document standardisation
- If applicable then, horizontal deployment.
Now I am going to discuss here some popular Manufacturing examples in different operations like a laboratory, quality, customer complaints, process issues or problems, etc.
CAPA Example in Laboratory:
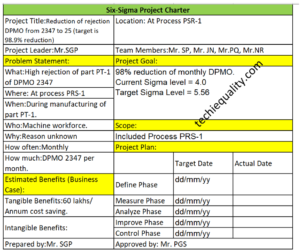
Let’s consider that a wrong TC (test certificate) has been generated at the laboratory and the same TC was communicated to the customer. For the same case, you are supposed to submit the CAPA report.
Process: laboratory operation.
Item: PQR, Date: dd/mm/yy.
Identification of problem: Wrong TC had been generated and communicated to the customer
Correction/ Containment Action: Checked the other TC & verified that all TC are OK, Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Why-Why Analysis or 5 Whys Analysis:
Why1: Wrong TC had been generated
Why2: Human Error
Why3: An Intermediate checking system was not available.
RC (Root cause): An Intermediate checking system was not available.
Corrective Action Plan:
Corrective Action: Intermediate checking system will be developed, Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Preventive Action Plan: [1] Preventive Action: Refresh Training, [2] Two layers checking of TC before releasing to customer. Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Verification of Implemented Action Plan: Checked that SOP has been made and implemented in the lab, Verified by: Mr. P.
Effectiveness Monitoring Status: Monitoring the monthly TC Status of OK or not OK, Remarks: No such error found in the last 3 months report.
Document updation / Standardization:
- SOP: X
- Check sheet:
- Control Plan: X
- FMEA: X
- R&P record: X
- Others:
Remarks: SOP has been made, Control plan, PFMEA, R&P has been updated w.r.t action plan.

Customer Complaint CAPA Report:
If you received any customer complaint and your customer is asking to submit the action plan and they have not mentioned any specified format then you can use the CAPA format or 8D report template for submitting the action plan. But many customers use the digital platform as SRM- supply relationship management portal to upload the action plan in their specified template.
You can follow the above-mentioned steps and can do refer to the above example for formulating the CAPA report in any type of customer complaint. Nevertheless, we are taking a simple customer complaint example for CAPA report formation.
Let’s consider a company manufacturing the automobile part and selling it to OEM customers. Unfortunately, a customer complaint was received due to a machining issue of a part PpP.
Process: Moulding operation
Item: PpP, Date: dd/mm/yy.
Identification of problem: Machining issue of a part PpP.
Correction/ Containment Action: Checked the other produced parts, inspected the dimensions and segregated the NG parts, Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Why-Why Analysis or 5 Whys Analysis:
Why1: machining issue of a part PpP.
Why2: The machining dimension was less as per the drawing.
Why 3: The core setting at mould operation was not adequate.
Why3: New operator
RC (Root cause): Lack of knowledge of new operators
Corrective Action Plan:
Corrective Action: On-job training will be imparted to new operators, Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Preventive Action Plan: [1] Periodic training evaluation [2] Training effectiveness monitoring [3] Training feedback. Target Date: dd/mm/yy, Responsibility: Mr. SGP.
Verification of Implemented Action Plan: training has been imparted to the operator and the skill matrix is being monitored for performance measurement of operators, verified by: Mr. P.
Effectiveness Monitoring Status: Monitoring the monthly operator’s skill matrix for performance measurement, Remarks: No such error found in the last 3 months.
Document updation / Standardization:
- Training SOP:
- Check sheet:
- Control Plan:
- FMEA:
- R&P record:
- Skill matrix.
- 4M Change.
Remarks: The above documents are updated but these are not limited to.
I hope the concept is clearly understood and now you can easily prepare the CAPA report.
QA/QC/TQM/Lean/6-Sigma Template or Format:
Useful Post:
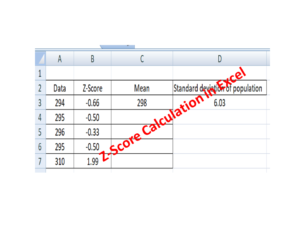
How to calculate z-score in excel with manufacturing example.
How to make a box plot in excel| Manufacturing Example
How to create forecast in excel | Illustration with Example
How to Choose the Best Forecasting Method| Marketing & Sales Example
More on TECHIEQUALITY